35 POINTS
Consider the following reaction:
4P + 502 → P4010
The percent yield of P4010...

Chemistry, 11.03.2021 19:30 dontcareanyonemo
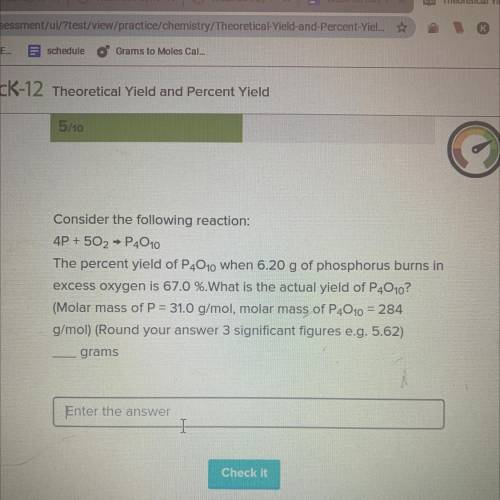
35 POINTS
Consider the following reaction:
4P + 502 → P4010
The percent yield of P4010 when 6.20 g of phosphorus burns in
excess oxygen is 67.0 %.What is the actual yield of P4010?
(Molar mass of P = 31.0 g/mol, molar mass of P4010 = 284
g/mol) (Round your answer 3 significant figures e. g. 5.62)
grams

Answers: 1
Another question on Chemistry



Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1

Chemistry, 22.06.2019 12:00
What is the subscript for oxygen in its molecular formula
Answers: 1
You know the right answer?
Questions


Chemistry, 27.01.2020 19:31

Mathematics, 27.01.2020 19:31


Mathematics, 27.01.2020 19:31


Computers and Technology, 27.01.2020 19:31



English, 27.01.2020 20:31


Social Studies, 27.01.2020 20:31


Computers and Technology, 27.01.2020 20:31


Mathematics, 27.01.2020 20:31



History, 27.01.2020 20:31



