
Chemistry, 11.03.2021 20:00 07corcum85504
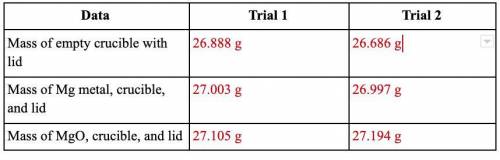
40 Points! Magnesium is the limiting reactant in this experiment. Calculate the theoretical yield of MgO for each trial.
Equation: Mg + O2 → MgO
This is the actual yield of magnesium oxide for each trial.
Trial 1: 0.217 g
Trial 2: 0.508 g

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 04:40
In which environment would primary succession occur? a forest with a few remaining trees after a recent wildfire an area of exposed rock after a glacier melts away beach that is exposed to the air at low tide an abandoned baseball field in a small town
Answers: 1

Chemistry, 22.06.2019 05:50
What happens when the temperature of a solution increases?
Answers: 2

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 3

Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1
You know the right answer?
40 Points! Magnesium is the limiting reactant in this experiment. Calculate the theoretical yield of...
Questions

Mathematics, 23.04.2021 01:50


Biology, 23.04.2021 01:50

English, 23.04.2021 01:50

History, 23.04.2021 01:50







Mathematics, 23.04.2021 01:50

Spanish, 23.04.2021 01:50


Mathematics, 23.04.2021 01:50


Mathematics, 23.04.2021 01:50

Mathematics, 23.04.2021 01:50


Mathematics, 23.04.2021 01:50



