
Chemistry, 11.03.2021 21:40 abdullahs4639
PLEASE WILL GIVE BRAINLIESTASAP
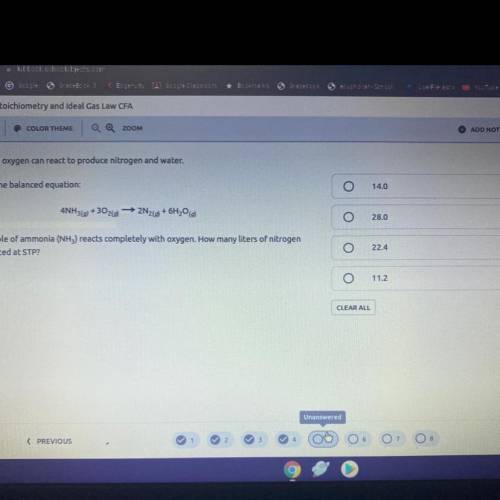
5. Ammonia and oxygen can react to produce nitrogen and water.
Following is the balanced equation:
4NH3(0)
+302(0)
- 2N269 + 6H20
Suppose 1 mole of ammonia (NH3) reacts completely with oxygen. How many liters of nitrogen
will be produced at STP?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:00
Chemical energy is a form of a. kinetic energy only. b. both potential and kinetic energy. c. neither potential nor kinetic energy. d. potential energy only. reset
Answers: 1


Chemistry, 22.06.2019 11:30
Compare and contrast refraction of light and sound will give brainliest
Answers: 1

Chemistry, 22.06.2019 18:30
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2
You know the right answer?
PLEASE WILL GIVE BRAINLIESTASAP
5. Ammonia and oxygen can react to produce nitrogen and water.
Questions

Mathematics, 12.03.2021 04:00





Mathematics, 12.03.2021 04:00

Mathematics, 12.03.2021 04:00

Biology, 12.03.2021 04:00

Mathematics, 12.03.2021 04:00

Physics, 12.03.2021 04:00

Advanced Placement (AP), 12.03.2021 04:00



History, 12.03.2021 04:00






Social Studies, 12.03.2021 04:00



