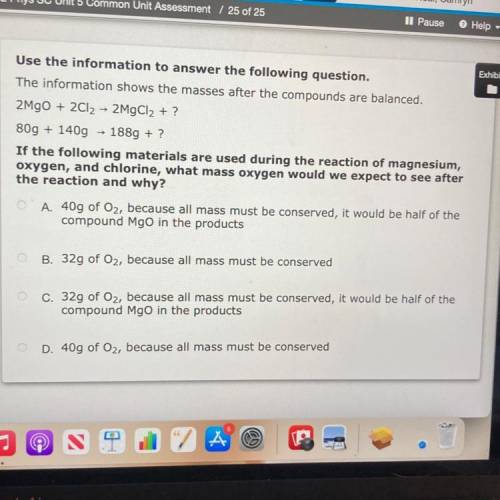
The information shows the masses after the compounds are balanced.
2MgO + 2Cl2 → 2MgCl2 + ?
8...

Chemistry, 11.03.2021 22:30 luthfipadasseri
The information shows the masses after the compounds are balanced.
2MgO + 2Cl2 → 2MgCl2 + ?
80g + 140g + 1889 + ?
If the following materials are used during the reaction of magnesium,
oxygen, and chlorine, what mass oxygen would we expect to see after
the reaction and why?
O A. 40g of Oz, because all mass must be conserved, it would be half of the
compound Mgo in the products
O B. 32g of Oz, because all mass must be conserved
c. 32g of Oz, because all mass must be conserved, it would be half of the
compound Mgo in the products
D. 40g of Oz, because all mass must be conserved

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:00
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3

Chemistry, 22.06.2019 03:00
Which step in naming unsaturated hydrocarbons is used for alkenes but not alkynes
Answers: 2

Chemistry, 22.06.2019 05:30
What is the mass defect of a mole of nuclei with 1.8 x 10^15 j/mol binding energy?
Answers: 1

You know the right answer?
Questions



Mathematics, 01.05.2021 01:30



Biology, 01.05.2021 01:30


Chemistry, 01.05.2021 01:30



Mathematics, 01.05.2021 01:30

English, 01.05.2021 01:30






English, 01.05.2021 01:30

Mathematics, 01.05.2021 01:30



