
Chemistry, 12.03.2021 01:20 angeisthe72
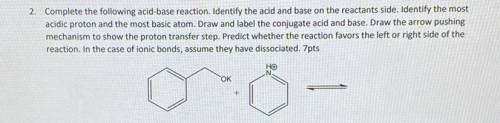
2. Complete the following acid-base reaction. Identify the acid and base on the reactants side. Identify the most
acidic proton and the most basic atom. Draw and label the conjugate acid and base. Draw the arrow pushing
mechanism to show the proton transfer step. Predict whether the reaction favors the left or right side of the
reaction. In the case of ionic bonds, assume they have dissociated.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 01:40
Brainliesttt me asap! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 22.06.2019 02:10
26. of of (aq) by (aq) is . if 50.00 ml of 1.05 m is to 25.00 ml of 1.86 m ,at be? ( no is toina of aof) , h.. (p. ). . .
Answers: 3

Chemistry, 22.06.2019 19:40
What is the wavelength of a 3*10^12 hz infrared wave a 3*10^20m b 1* 10^4m c 3*10^-3m d 1*10^-4 m
Answers: 1

Chemistry, 22.06.2019 21:30
Liquid ammonia is produced at high temperatures and under great pressure in a tank by passing a mixture of nitrogen gas and hydrogen gas over an iron catalyst. the reaction is represented by the following equation. n2(g) + 3h2(g) → 2nh3(g) changing all but one experimental condition will affect the amount of ammonia produced. that condition is a) increasing the concentration of both reactants b) changing the temperature within the tank c) decreasing the pressure within the tank. d) increasing only the amount of nitrogen present.
Answers: 1
You know the right answer?
2. Complete the following acid-base reaction. Identify the acid and base on the reactants side. Iden...
Questions

Social Studies, 30.01.2020 04:02

Social Studies, 30.01.2020 04:02


Mathematics, 30.01.2020 04:02


History, 30.01.2020 04:02



History, 30.01.2020 04:02


Mathematics, 30.01.2020 04:02


Biology, 30.01.2020 04:02


Social Studies, 30.01.2020 04:02


Mathematics, 30.01.2020 04:02



Geography, 30.01.2020 04:02



