C2H4(g) + 3O2(g) —> 2CO2(g) + 2H2O(g)
change in H1 = ?
The combustion of C2H4 is rep...

Chemistry, 12.03.2021 03:00 rustjallison9928
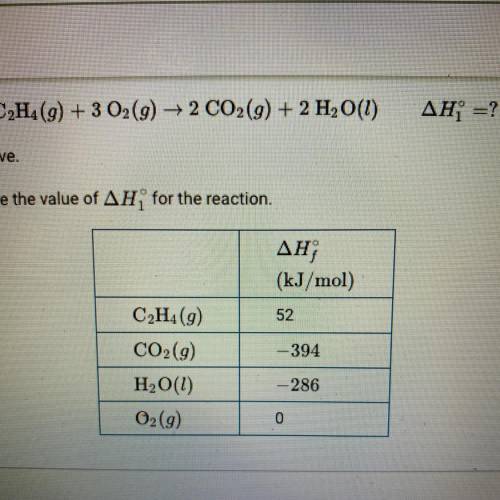
C2H4(g) + 3O2(g) —> 2CO2(g) + 2H2O(g)
change in H1 = ?
The combustion of C2H4 is represented by the equation above.
Use the enthalpies of formation in the table the calculate the value of change in H1 for the reaction.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 14:50
What type of reaction is illustrated? 2c12o5 = 2cl2 + 502
Answers: 2

Chemistry, 22.06.2019 05:20
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

Chemistry, 22.06.2019 05:40
Why did southern business leaders want to increase the number of slaves
Answers: 1

Chemistry, 22.06.2019 06:00
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2
You know the right answer?
Questions


English, 07.12.2020 05:40



English, 07.12.2020 05:40

Advanced Placement (AP), 07.12.2020 05:40

Health, 07.12.2020 05:40



Mathematics, 07.12.2020 05:40

History, 07.12.2020 05:40

Physics, 07.12.2020 05:40

Mathematics, 07.12.2020 05:40


English, 07.12.2020 05:40

English, 07.12.2020 05:40


Health, 07.12.2020 05:40

History, 07.12.2020 05:40



