
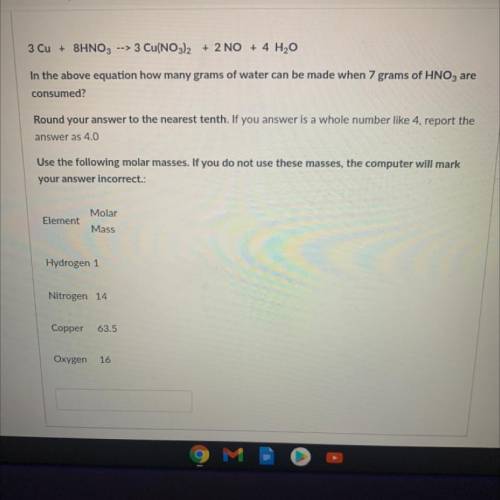
3 Cu + 8HNO3 --> 3 Cu(NO3)2 + 2 NO + 4 H2O
In the above equation how many grams of water can be made when 7 grams of HNO3 are
consumed?
Round your answer to the nearest tenth. If you answer is a whole number like 4, report the
answer as 4.0
Use the following molar masses. If you do not use these masses, the computer will mark
your answer incorrect.:
Element
Molar
Mass
Hydrogen 1
Nitrogen 14
Copper
63.5
Oxygen
16

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:00
At room temperature what happens to the average kinetic energy of the molecules of a solid, liquid, and a gas
Answers: 2

Chemistry, 22.06.2019 03:30
The atomic radius of sodium is 186 pm and of chlorine is 100 pm. the ionic radius for na+ is 102 pm and for cl– is 181 pm. in going from na to cl in period 3, why does the atomic radius decrease while the ionic radius increases? a. the inner electrons in the sodium cation shield its valence electrons more effectively than the inner electrons in the chloride anion do. b. the inner electrons shield the valence electrons more effectively in the chlorine atom than in the chloride anion. c. the outermost electrons in chloride experience a smaller effective nuclear charge than those in the sodium cation do. d. the outermost electrons in chloride experience a larger effective nuclear charge than those in the sodium cation do. e. monatomic ions are bigger than the atoms from which they are formed.
Answers: 2

Chemistry, 23.06.2019 02:00
Which best describes the present-day universe? opaque, expanding very slowly, stars produce heavy elements transparent, expanding at an accelerated rate, stars produce heavy elements opaque, expanding at an accelerated rate, stars produce only hydrogen and helium transparent, expanding very slowly, stars produce only hydrogen and helium
Answers: 1

Chemistry, 23.06.2019 02:00
When an experimenter draws a conclusion that he assumes will apply to all situations set up similarly to his test situation, even though he cannot possibly have examined all possible test scenarios, the experimenter is using deductive reasoning inductive reasoning abductive reasoning subjective reasoning
Answers: 1
You know the right answer?
3 Cu + 8HNO3 --> 3 Cu(NO3)2 + 2 NO + 4 H2O
In the above equation how many grams of water can be...
Questions




History, 24.08.2020 01:01

Health, 24.08.2020 01:01

History, 24.08.2020 01:01


Mathematics, 24.08.2020 01:01


Mathematics, 24.08.2020 01:01

History, 24.08.2020 01:01

Mathematics, 24.08.2020 01:01

Mathematics, 24.08.2020 01:01


English, 24.08.2020 01:01

Mathematics, 24.08.2020 01:01


Mathematics, 24.08.2020 01:01

Mathematics, 24.08.2020 01:01



