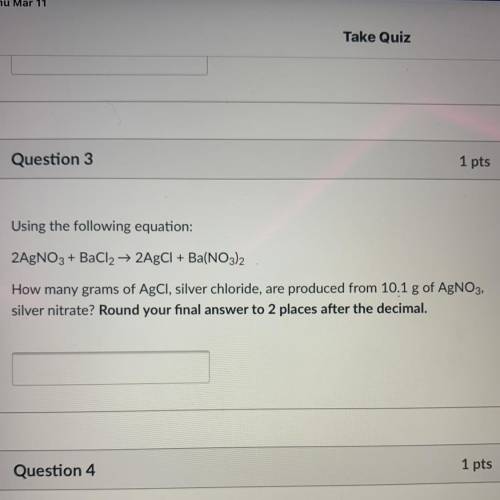
Using the following equation:
2AgNO3 + BaCl2 + 2AgCl + Ba(NO3)2
How many grams of AgCl, silve...


Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Which best describes why nh4+ can form an ionic bond with ci-?
Answers: 1


Chemistry, 23.06.2019 01:30
The biomedical technique in which a part of the brain is destroyed with electric current is known as a. electroconvulsive therapy b. prefrontal lobotomy c. bilateral cingulotomy d. tardive dyskinesia
Answers: 2

Chemistry, 23.06.2019 07:40
)in the deacon process for the manufacture of chlorine, hcl and o2 react to form cl2 and h2o. sufficient air (21 mole% o2, 79% n2) is fed to provide 35% excess oxygen, and the fractional conversion of hcl is 85%. calculate the mole fractions of the product stream components.
Answers: 1
You know the right answer?
Questions

Health, 10.12.2020 01:40

History, 10.12.2020 01:40

History, 10.12.2020 01:40

Physics, 10.12.2020 01:40

Mathematics, 10.12.2020 01:40

Physics, 10.12.2020 01:40



Mathematics, 10.12.2020 01:40





Mathematics, 10.12.2020 01:40



English, 10.12.2020 01:40


Chemistry, 10.12.2020 01:40