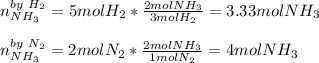If you have 5 mol H2 and 2 mol N2, what is the limiting reagent in the reaction
below?
3 H2(g...

Chemistry, 12.03.2021 08:40 gabbym39077
If you have 5 mol H2 and 2 mol N2, what is the limiting reagent in the reaction
below?
3 H2(g) + N2 (g) → 2 NH3 (g)

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:00
Of the groups of elements below, which are most likely to gain electrons to become anions? a. alkali metal b. boron group c. halogen d. transition metal
Answers: 2

Chemistry, 22.06.2019 04:30
What are the primary responsibilities of a chemical engineer involved in "r& d"? develop large scale manufacturing operations discover new products and processes training of new chemists determine products needed by consumers
Answers: 2

Chemistry, 22.06.2019 14:30
Amixture that has two or more substances that are spread out evenly is called a. compound b. heterogeneous c. substance d. homogeneous
Answers: 1

Chemistry, 22.06.2019 14:50
Consider the following multistep reaction: a b→ab(slow) a ab→a2b(fast)−−−−−−−−−−−−−−−−− 2a b→a2b(overall) based on this mechanism, determine the rate law for the overall reaction. express your answer in standard masteringchemistry format. for example, if the rate law is k[a]3[b]2 type k*[a]^3*[b]^2
Answers: 3
You know the right answer?
Questions

English, 15.04.2020 19:08






Physics, 15.04.2020 19:08


Biology, 15.04.2020 19:08

Physics, 15.04.2020 19:08