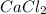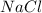Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Iknow the answer to 13 is b and 14 is d. i just need to know why the correct answers are correct
Answers: 3

Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1

Chemistry, 22.06.2019 21:30
What is the effect of returning nuclear reactor cooling water back into bodies of water?
Answers: 3

You know the right answer?
2 moles of sodium phosphate reacts with 3 moles of
calcium chloride
2Na3PO4 + 3 CaCl2 -> 6...
2Na3PO4 + 3 CaCl2 -> 6...
Questions

Mathematics, 22.06.2019 01:30

Mathematics, 22.06.2019 01:30

Mathematics, 22.06.2019 01:30


Mathematics, 22.06.2019 01:30





Mathematics, 22.06.2019 01:30










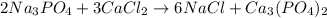
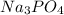 require 3 moles of
require 3 moles of