
Chemistry, 12.03.2021 15:20 pickelswolf3962
Nickel carbonyl decomposes to form nickel and carbon monoxide, like this:
Ni(CO)4(g) → Ni(s)+ 4CO(g)
At a certain temperature, a chemist finds that a 2.6 L reaction vessel containing a mixture of nickel carbonyl, nickel, and carbon monoxide at equilibrium has the following composition:
compound amount
Ni(CO)4 0.597g
Ni 12.7g
CO 1.98g
Required:
Calculate the value of the equilibrium constant for this reaction.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:40
Which characteristic of water it form droplets? a. low specific heat b. nonpolar structure c. high surface tension d. ability to dissolve substances
Answers: 1

Chemistry, 22.06.2019 06:00
Oxidation-reduction reactions (often called "redox" for short) are reactions that involve the transfer of electrons from one species to another. oxidation states, or oxidation numbers, allow chemists to keep track of these electron transfers. in general, one element will lose electrons (oxidation), with the result that it will increase in oxidation number, and another element will gain electrons (reduction), thereby decreasing in oxidation number. the species that is oxidized is called the reducing agent or reductant. the species that is reduced is called the oxidizing agent or oxidant. to sum up: oxidation = increase in oxidation state = loss of electrons = reducing agent reduction = decrease in oxidation state = gain of electrons = oxidizing agent part a which element is oxidized in this reaction? fe2o3+3co→2fe+3co2 enter the elemental symbol. view available hint(s) is oxidized part b which element is reduced in this reaction? 2hcl+2kmno4+3h2c2o4→6co2+2mno2+2kcl+4h2o enter the elemental symbol. view available hint(s) is reduced
Answers: 1

Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2

Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1
You know the right answer?
Nickel carbonyl decomposes to form nickel and carbon monoxide, like this:
Ni(CO)4(g) → Ni(s)+ 4CO(g...
Questions


Biology, 01.12.2020 07:40


History, 01.12.2020 07:40


Mathematics, 01.12.2020 07:40

Mathematics, 01.12.2020 07:40

Geography, 01.12.2020 07:40

Advanced Placement (AP), 01.12.2020 07:40

Mathematics, 01.12.2020 07:40

Mathematics, 01.12.2020 07:40


Mathematics, 01.12.2020 07:40


Mathematics, 01.12.2020 07:40


Mathematics, 01.12.2020 07:40

Mathematics, 01.12.2020 07:40


Mathematics, 01.12.2020 07:40

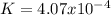
![K=\frac{[CO]^4}{[Ni(CO)_4]}](/tpl/images/1190/9822/9636a.png)
![[CO]_{EQ}=\frac{1.98g}{28.01g/mol} *\frac{1}{2.6L}=0.0272M](/tpl/images/1190/9822/0dde7.png)
![[Ni(CO)_4]_{EQ}=\frac{0.597g}{170.73g/mol} *\frac{1}{2.6L}=0.001345M](/tpl/images/1190/9822/2339a.png)



