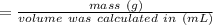Chemistry, 12.03.2021 15:30 alisonlebron15
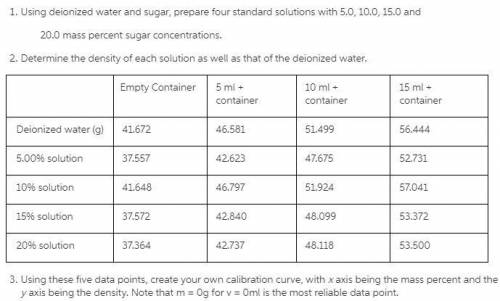
Using deionized water and sugar, prepare four standard solutions with 5.0, 10.0, 15.0 and 20.0 mass percent sugar concentrations. Determine the density of each solution as well as that of the deionized water.
Empty Container 5 ml + container 10 ml + container 15 ml + container
Deionized water (g) 41.672 46.581 51.499 56.444
5.00% solution 37.557 42.623 47.675 52.731
10% solution 41.648 46.797 51.924 57.041
15% solution 37.572 42.840 48.099 53.372
20% solution 37.364 42.737 48.118 53.500
Using these five data points, create your own calibration curve, with x axis being the mass percent and the y axis being the density.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 23:50
How does a scientist the government? a. the scientist tells people in society what to do. b. the scientist determines the policies that the government spends money on. c. the scientist provides unbiased information to the government. d. the scientist makes laws based on his or her research results.
Answers: 1

Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1
You know the right answer?
Using deionized water and sugar, prepare four standard solutions with 5.0, 10.0, 15.0 and 20.0 mass...
Questions

Chemistry, 12.10.2020 03:01


Mathematics, 12.10.2020 03:01

Chemistry, 12.10.2020 03:01

World Languages, 12.10.2020 03:01

History, 12.10.2020 03:01


Physics, 12.10.2020 03:01


History, 12.10.2020 03:01

Spanish, 12.10.2020 03:01

World Languages, 12.10.2020 03:01

English, 12.10.2020 03:01

History, 12.10.2020 03:01



Computers and Technology, 12.10.2020 03:01

Health, 12.10.2020 03:01


History, 12.10.2020 03:01