Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 07:00
At 450 mm hg a gas has a volume of 760 l, what is its volume at standard pressure
Answers: 2

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 2


You know the right answer?
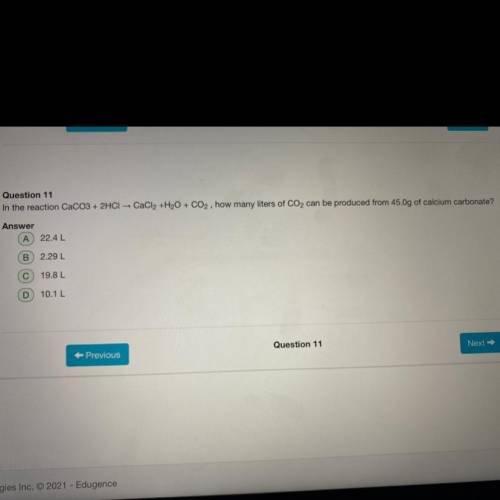
In the reaction CaCO3+2HCl CaCl 2 +H 2 O+CO 2 how many liters of CO 2 can be produced from 45.0g of...
Questions




Physics, 16.10.2019 21:30


History, 16.10.2019 21:30




Mathematics, 16.10.2019 21:30


English, 16.10.2019 21:30

Business, 16.10.2019 21:30



Geography, 16.10.2019 21:30

Chemistry, 16.10.2019 21:30

History, 16.10.2019 21:30

Chemistry, 16.10.2019 21:30

Biology, 16.10.2019 21:30