
Chemistry, 13.03.2021 01:00 mvtthewisdead
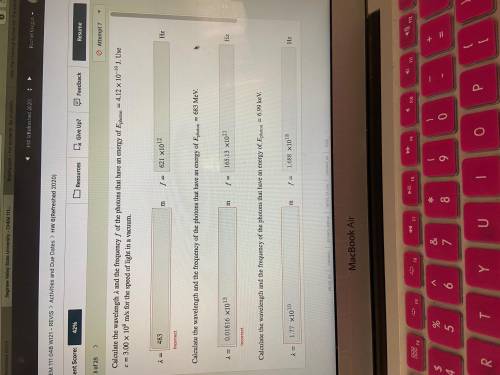
Calculate the wavelength and the frequency of the photons that have an energy of photon=4.12×10−19 J. Use =3.00×108 m/s for the speed of light in a vacuum.
Calculate the wavelength and the frequency of the photons that have an energy of photon=683 MeV.
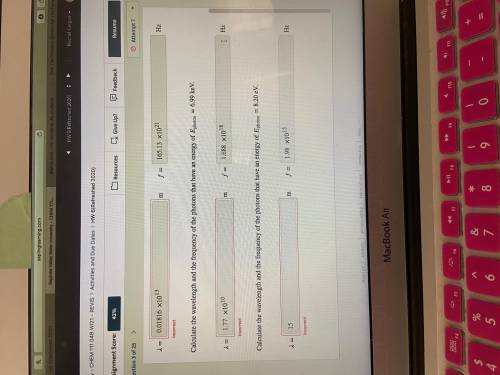
Calculate the wavelength and the frequency of the photons that have an energy of photon=6.99 keV.
Calculate the wavelength and the frequency of the photons that have an energy of photon=8.20 eV.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Asmall amount of a solid is added to water. the observation made after fifteen minutes is shown in the figure. which of these solids has been probably added to water? a) oil b) sand c) sugar d) wood chips
Answers: 1


Chemistry, 23.06.2019 02:40
Calculate the standard enthalpy of formation of liquid methanol, ch3oh(l), using the following information: c(graphite) + o2 latex: \longrightarrow ⟶ co2(g) latex: \delta δ h° = –393.5 kj/mol h2(g) + o2 latex: \longrightarrow ⟶ h2o(l) latex: \delta δ h° = –285.8 kj/mol ch3oh(l) + o2(g) latex: \longrightarrow ⟶ co2(g) + 2h2o(l) latex: \delta δ h° = –726.4 kj/mol
Answers: 3

Chemistry, 23.06.2019 04:31
What is the amount of energy for a photon that has a 125 cm wavelength
Answers: 2
You know the right answer?
Calculate the wavelength and the frequency of the photons that have an energy of photon=4.12×10−19 J...
Questions

Mathematics, 18.06.2021 04:50

English, 18.06.2021 04:50



Mathematics, 18.06.2021 04:50




Arts, 18.06.2021 04:50



Business, 18.06.2021 04:50



Business, 18.06.2021 04:50


Mathematics, 18.06.2021 04:50


Mathematics, 18.06.2021 04:50




