
Chemistry, 13.03.2021 04:00 youngcie04
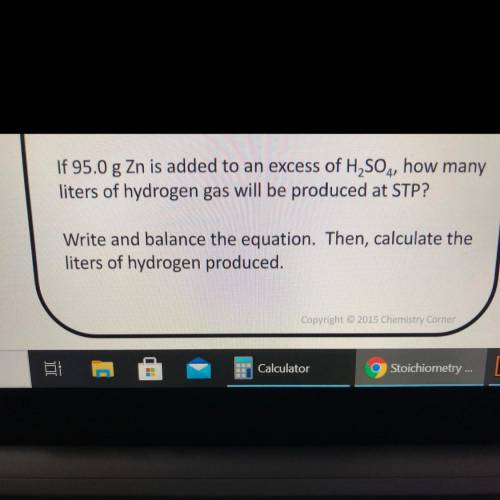
If 95.0 g Zn is added to an excess of H2SO4, how many
liters of hydrogen gas will be produced at STP?
Write and balance the equation. Then, calculate the
liters of hydrogen produced.
Please help

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 22:00
The graph above shows how the price of cell phones varies with the demand quantity. the equilibrium price for cell phones is where both supply and demand quantities equal $100, 5,000 5,000, $100
Answers: 2

Chemistry, 22.06.2019 05:00
Given sno2 + 2h2 - sn + 2h20 tin oxide reacts with hydrogen to produce tin and water. how many moles of sno2 are needed to produce 500.0 grams of sn?
Answers: 3

Chemistry, 22.06.2019 05:30
What is the mass of each element in a 324.8 sample of co2
Answers: 1

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1
You know the right answer?
If 95.0 g Zn is added to an excess of H2SO4, how many
liters of hydrogen gas will be produced at ST...
Questions



History, 23.03.2021 01:20




Mathematics, 23.03.2021 01:20

Mathematics, 23.03.2021 01:20

Spanish, 23.03.2021 01:20


Arts, 23.03.2021 01:20

Mathematics, 23.03.2021 01:20


Mathematics, 23.03.2021 01:20


English, 23.03.2021 01:20

Chemistry, 23.03.2021 01:20





