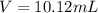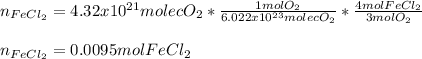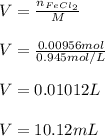Chemistry, 13.03.2021 04:20 carrottopsadly
For the reaction 4 FeCl2(aq) + 302(g) → 2Fe2O3(s) + 4Cl2(g), what volume of a
0.945 M solution of FeCl2 is required to react completely with 4.32 x1021 molecules
of O2?
4.23 x 103 mL
09.04 mL
O 5.69 mL
O 10.1 mL
O 5.08 mL

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 03:00
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3


Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2
You know the right answer?
For the reaction 4 FeCl2(aq) + 302(g) → 2Fe2O3(s) + 4Cl2(g), what volume of a
0.945 M solution of F...
Questions

Mathematics, 11.01.2021 18:30

Mathematics, 11.01.2021 18:30


History, 11.01.2021 18:30



English, 11.01.2021 18:30

Mathematics, 11.01.2021 18:30

Social Studies, 11.01.2021 18:30




Mathematics, 11.01.2021 18:30


Mathematics, 11.01.2021 18:30

History, 11.01.2021 18:30


Mathematics, 11.01.2021 18:30

Mathematics, 11.01.2021 18:30