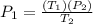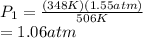Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Asample of neon occupies a volume of 375 ml at stp. what will be the volume of neon if the pressure is reduced to 90.0 kpa? a. 422 ml b. 422 l c. 333 ml d. 333 l
Answers: 2

Chemistry, 22.06.2019 04:20
Which of the following is true for the actual yield of a reaction? it is always calculated as a ratio. it is the yield from the excess reactant. it is the yield from the limiting reactant. it is always less than the theoretical yield.
Answers: 1

Chemistry, 22.06.2019 06:30
The minerals found in bones are deposited by living cells called
Answers: 1

Chemistry, 22.06.2019 08:00
This classification of drug typically changes the brain's chemistry and reduces its ability to create its own endorphins.
Answers: 1
You know the right answer?
A gas container has an initial temperature of 348 K with an unknown pressure. When the temperature c...
Questions



Social Studies, 31.08.2020 01:01



Engineering, 31.08.2020 01:01



Spanish, 31.08.2020 01:01

Chemistry, 31.08.2020 01:01






Physics, 31.08.2020 01:01

Mathematics, 31.08.2020 01:01

Mathematics, 31.08.2020 01:01


Mathematics, 31.08.2020 01:01

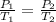
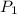 .
.