
Chemistry, 17.03.2021 23:40 brianna1096
A tank consists of a gaseous mixture of 3.83 g of triselenium diiodide, 2.62 g of ammonium telluride, and 1.82 g of iridium (i) sulfate. What is the partial pressure in atm of ammonium telluride if the total pressure in the tank is 2.795 atm?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 14:30
The length of a vector arrow represents its magnitude and the point represents its direction true or false apex
Answers: 3

Chemistry, 22.06.2019 06:30
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1

Chemistry, 22.06.2019 08:30
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 22.06.2019 10:50
8) a mixture of he, ne and ar has a pressure of 7.85 atm. if the ne has a mole fraction of 0.47 and 8) ar has a mole fraction of 0.23, what is the pressure of he? a) 4.2 atm b) 3.7 atm c) 5.5 atm d) 2.4 atm e) 1.8 atm
Answers: 1
You know the right answer?
A tank consists of a gaseous mixture of 3.83 g of triselenium diiodide, 2.62 g of ammonium telluride...
Questions

Social Studies, 20.09.2020 17:01




Computers and Technology, 20.09.2020 17:01

English, 20.09.2020 17:01




Mathematics, 20.09.2020 17:01




Mathematics, 20.09.2020 17:01

Mathematics, 20.09.2020 17:01



Mathematics, 20.09.2020 17:01

Geography, 20.09.2020 17:01

Law, 20.09.2020 17:01


 = partial pressure of A
= partial pressure of A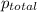 = total pressure
= total pressure  ,
, 



