
Chemistry, 17.03.2021 23:50 bella122805
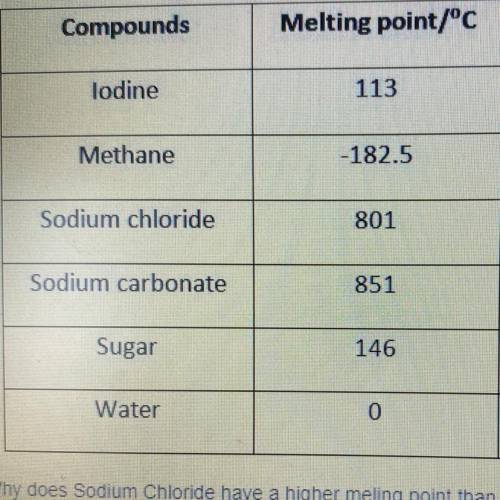
Why does Sodium Chloride have a higher melting point than Sugar?
a. intermolecular forces are weaker
b. intermolecular forces are slightly stronger
c. intermolecular forces are very strong
d. melting point is based on composition and not bonding

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 20:10
Starch and are common polysaccharide carbohydrates found in plants. sucrose glycogen fructose cellulose
Answers: 3

Chemistry, 22.06.2019 23:00
What is the measured amount of a product obtained from a chemical reaction?
Answers: 1

Chemistry, 23.06.2019 11:20
The chemical composition of soil varies with depth. an article in communications in soil science and plant analysis describes chemical analyses of soil taken from a farm in western australia. fifty specimens were each taken at depths 50 and 250 cm. at a depth of 50 cm, the average no3 concentration (in mg/l) was 88.5 with a standard deviation of 49.4. at a depth of 250 cm, the average concentration was 110.6 with a standard deviation of 51.5. find a 95% confidence interval for the difference in no3 concentrations at the two depths.
Answers: 1

Chemistry, 23.06.2019 13:30
Asap a 50.0 ml soap bubble is blown in a 27.0°c room. it drifts out an open window and lands in a snow bank at -3.0°c. what is its new volume?
Answers: 1
You know the right answer?
Why does Sodium Chloride have a higher melting point than Sugar?
a. intermolecular forces are weake...
Questions





History, 28.06.2019 02:20

History, 28.06.2019 02:20

Mathematics, 28.06.2019 02:20









Social Studies, 28.06.2019 02:20



Social Studies, 28.06.2019 02:20

Mathematics, 28.06.2019 02:20



