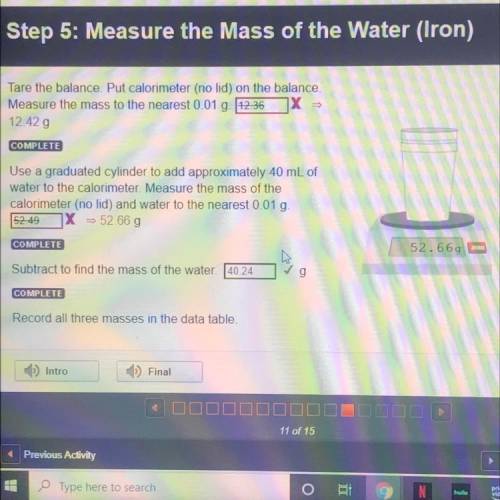
(STEP 5: IRON)
Tare the balance. Put calorimeter (no lid) on the balance.
Measure the mass to...

Chemistry, 17.03.2021 23:50 emmajobaby
(STEP 5: IRON)
Tare the balance. Put calorimeter (no lid) on the balance.
Measure the mass to the nearest 0.01 g
12.42 g
Use a graduated cylinder to add approximately 40 mL of
water to the calorimeter. Measure the mass of the
calorimeter (no lid) and water to the nearest 0.01 g.
52.66 g
Subtract to find the mass of the water. 40.24
g

Answers: 1
Another question on Chemistry



Chemistry, 23.06.2019 08:40
The activation energy for this reaction is 75 kj·mol–1. the enzyme catalase (found in blood) lowers the activation energy to 8.0 kj·mol–1. at what temperature would the non-catalyzed reaction need to be run to have a rate equal to that of the enzyme-catalyzed reaction at 25°c?
Answers: 2

Chemistry, 23.06.2019 18:10
Which is an aspect of the kinetic-molecular theory and can be used to explain the behavior of plasmas? particle spacing can allow a very high density. particle kinetic energy is independent of temperature. particles vibrate quickly in stationary positions. particles exchange energy through elastic collisions.
Answers: 2
You know the right answer?
Questions

Computers and Technology, 20.09.2020 16:01

Mathematics, 20.09.2020 16:01

Computers and Technology, 20.09.2020 16:01


Mathematics, 20.09.2020 16:01

Engineering, 20.09.2020 16:01



History, 20.09.2020 16:01


Mathematics, 20.09.2020 16:01

Mathematics, 20.09.2020 16:01


Chemistry, 20.09.2020 16:01

English, 20.09.2020 16:01




English, 20.09.2020 16:01

Chemistry, 20.09.2020 16:01



