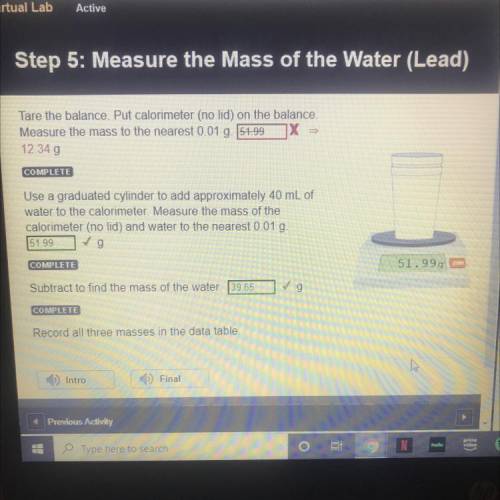
STEP 5: LEAD
Tare the balance. Put calorimeter (no lid) on the balance.
Measure the mass to t...

Chemistry, 17.03.2021 23:50 bartekpiglo
STEP 5: LEAD
Tare the balance. Put calorimeter (no lid) on the balance.
Measure the mass to the nearest 0.01 g.
12.34 g
Use a graduated cylinder to add approximately 40 mL of
water to the calorimeter. Measure the mass of the
calorimeter (no lid) and water to the nearest 0.01 g.
51.99 g
Subtract to find the mass of the water. 39.65 g

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 22:50
Select the correct answer how does the heat content of the reaction change in the process of photosynthesis when a glucose molecule is formed? ca the value of is negative the value of qis positive the value of a remains constant the value of a decreases the value of equals zero e
Answers: 2

Chemistry, 22.06.2019 00:00
Aside from human impact, which of the following causes less water vapor production over a small area? (2 pderivartin
Answers: 1

Chemistry, 22.06.2019 02:30
Margaret wants to make an orange flavored drink by stirring powdered drink mix into a glass of water. she doesn't like drinks that have small clumps of powdered solid in them, so she wants the drink to be a perfect solution. what factors should margaret not consider when deciding how much powder to add to her glass of water?
Answers: 3

Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2
You know the right answer?
Questions


Mathematics, 11.10.2019 06:00



History, 11.10.2019 06:00

Mathematics, 11.10.2019 06:00

Biology, 11.10.2019 06:00




Business, 11.10.2019 06:00

History, 11.10.2019 06:00



Biology, 11.10.2019 06:00




Mathematics, 11.10.2019 06:00



