
Chemistry, 17.03.2021 23:50 JamesLachoneus
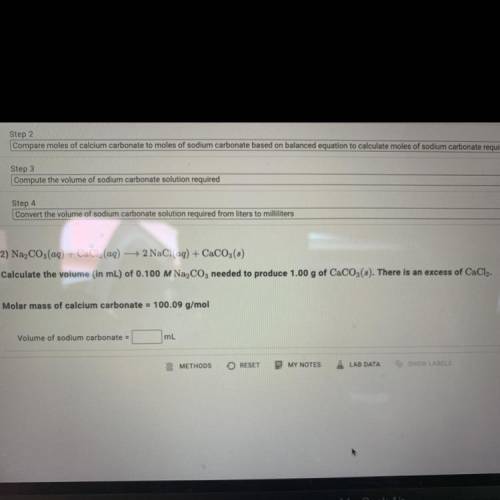
Calculate the volume (in mL) of 0.100 M Na, C03 needed to produce 1.00 g of CaCO3(s).
There is an excess of CaCl2.
Molar mass of calcium carbonate = 100.09 g/mol
Volume of sodium carbonate = ?mL

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:10
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3

Chemistry, 22.06.2019 12:50
The number at the end of an isotope’s name is the number.
Answers: 1


Chemistry, 22.06.2019 18:30
Which of the following nuclei would be the least stable a 2 protons, 2 neutrons b 1 proton 1 neutron c 1 proton 3 neutrons d 1 proton 2 neutrons
Answers: 3
You know the right answer?
Calculate the volume (in mL) of 0.100 M Na, C03 needed to produce 1.00 g of CaCO3(s).
There is an e...
Questions

Mathematics, 22.06.2019 23:00





History, 22.06.2019 23:00

Mathematics, 22.06.2019 23:00

Mathematics, 22.06.2019 23:00

Biology, 22.06.2019 23:00

English, 22.06.2019 23:00

Chemistry, 22.06.2019 23:00

Biology, 22.06.2019 23:00



Physics, 22.06.2019 23:00

English, 22.06.2019 23:00

Mathematics, 22.06.2019 23:00





