Chemistry, 18.03.2021 01:10 katherineweightman
A sample of aluminum, which has a specific heat capacity of 0.897·J·g−1°C^−1 , is put into a calorimeter (see sketch at right) that contains 300.0g of water. The aluminum sample starts off at 94.5°C and the temperature of the water starts off at 21.0°C .When the temperature of the water stops changing it's 23.8°C .The pressure remains constant at 1atm .Calculate the mass of the aluminum sample.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 16:00
Measuring which physical property is most likely to produce the most precise results when trying to identify a substance
Answers: 1

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 22.06.2019 19:50
Which sentence from holes contains an implied personality trait? stanley and his parents had tried to pretend that he was just going away to camp for a while, just like rich kids do. he'd just been in the wrong place at the wrong time. stanley felt somewhat dazed as the guard unlocked his handcuffs and led him off the bus. stanley nodded to show he understood
Answers: 3
You know the right answer?
A sample of aluminum, which has a specific heat capacity of 0.897·J·g−1°C^−1 , is put into a calorim...
Questions



Physics, 12.03.2021 03:10









Health, 12.03.2021 03:20

Mathematics, 12.03.2021 03:20





Mathematics, 12.03.2021 03:20

Mathematics, 12.03.2021 03:20

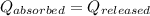
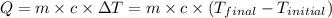
![m_1\times c\times (T_{final}-T_1)=-[m_2\times c\times (T_{final}-T_2)]](/tpl/images/1195/8097/b0e58.png)
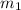 = mass of aluminium = ?
= mass of aluminium = ?
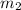 = mass of water = 300.0 g
= mass of water = 300.0 g
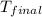 = final temperature =
= final temperature = 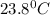
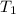 = temperature of aluminium =
= temperature of aluminium = 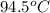
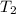 = temperature of water =
= temperature of water = 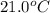
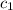 = specific heat of aluminium =
= specific heat of aluminium = 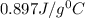
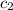 = specific heat of water =
= specific heat of water = 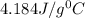
![-[m_1\times c_1\times (T_{final}-T_1)]=[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/1195/8097/92a72.png)
![-[m_1\times 0.897\times (23.8-94.5)^0C]=[300.0g\times 4.184\times (23.8-21.0)]](/tpl/images/1195/8097/6f259.png)