
Chemistry, 18.03.2021 01:20 trvptierra
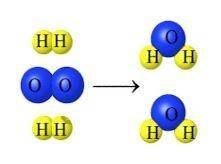
What evidence does the image below give for the Law of Conservation of Mass?
A. The number of hydrogen and oxygen is the same for the reactants and products.
B. The hydrogen and oxygen bonded together to create an new substance.
C. The hydrogen and oxygen go through a synthesis reaction to form H2O.
D. They chemically form a substance using the reactants to make a brand new product.

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 20:30
What was the procedure by which case united states vs lopez went to court
Answers: 1

Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3

Chemistry, 22.06.2019 22:30
Rank the four gases (air, exhaled air, gas produced from from decomposition of h2o2, gas from decomposition of nahco3) in order of increasing concentration of co2
Answers: 1

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 1
You know the right answer?
What evidence does the image below give for the Law of Conservation of Mass?
A. The number of hydro...
Questions








Computers and Technology, 24.01.2020 04:31









History, 24.01.2020 04:31


English, 24.01.2020 04:31

Biology, 24.01.2020 04:31



