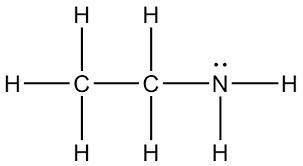
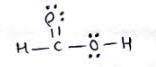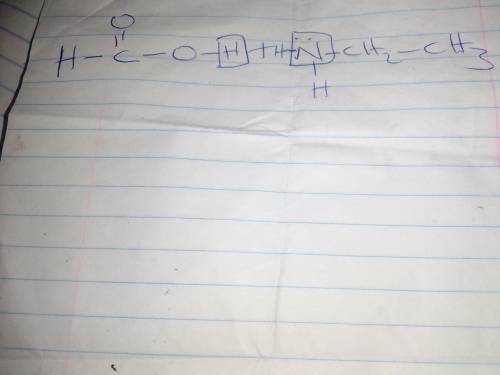Consider the reaction that occurs between the acid CHOOH and the base CH3CH2NH2.
A) Draw the complete Lewis structures for the acid and the base.
B) Explain why OOH is more acidic than CH3CH2NH2.
C) Explain why CH3CH2NH2 is more basic than CHOOH.
D) Re-draw your Lewis structures from part a. Add to these structures to show why these two molecules are attracted to each other. Do not show a curved arrow mechanism here.
E) Using curved arrows, show the reaction mechanism for the reaction of CHOOH with CH3CH2NH2.
F. Using the Lewis definition of acid-base reactions, explain what is happening at the molecular level during this reaction.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:20
Neils bohr believed that electrons orbited the nucleus in different energy levels, based on strong support from
Answers: 1

Chemistry, 22.06.2019 05:30
What happens to the atomic radius when an elctron is lost
Answers: 1

Chemistry, 22.06.2019 11:20
Which of the following contributes to the structural rigidity of cellulose? adjacent glucose polymers are stabilized by hydrogen bonding. glucose residues are joined by (α1→4) linkages. cellulose is a highly branched molecule. the conformation of the glucose polymer is a coiled structure.
Answers: 2

Chemistry, 22.06.2019 20:30
What is a difference between a mixture of elements and a mixture of compounds
Answers: 1
You know the right answer?
Consider the reaction that occurs between the acid CHOOH and the base CH3CH2NH2.
A) Draw the comple...
Questions

History, 01.07.2019 21:00



Computers and Technology, 01.07.2019 21:00

Arts, 01.07.2019 21:00

History, 01.07.2019 21:00

Mathematics, 01.07.2019 21:00


History, 01.07.2019 21:00





Mathematics, 01.07.2019 21:00


Biology, 01.07.2019 21:00


Chemistry, 01.07.2019 21:00

Mathematics, 01.07.2019 21:00

Mathematics, 01.07.2019 21:00