
Chemistry, 18.03.2021 01:20 juliayaccarinoo
If the pressure exerted by a gas at 100°C in a volume of 8.04 L is 3.81 atm, how many moles of gas are present
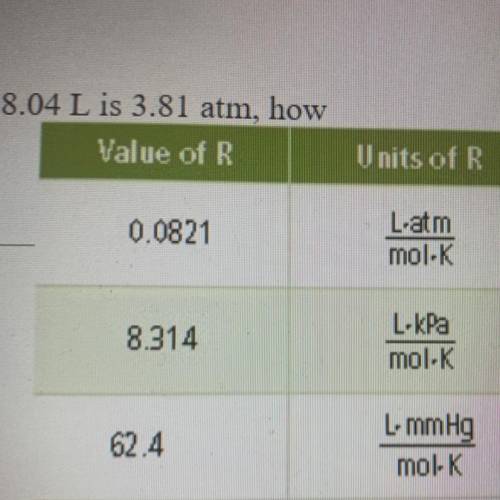

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 23:00
Movement that is like a t a type of wave that transfers energy where the particles in the medium move in a circle motion while the energy travels left or right. a type of wave that transfers energy where the particles in the medium move perpendicular to the direction in which the energy is traveling. transfers energy from one location to another a type of wave that transfers energy where the particles in the medium move parallel to the direction in which the energy is traveling. movement that is back and forth, like an equal sign = 1. wave 2. parallel movement 3. perpendicular movement 4. transverse wave 5. longitudinal wave 6. surface wave
Answers: 1

Chemistry, 23.06.2019 04:31
Areaction is first order. if the initial reactant concentration is 0.0200 m, and 25.0 days later the concentration is 6.25 x 10-4 m, then its half-life is:
Answers: 1

Chemistry, 23.06.2019 04:40
[01.07]what is the answer to the problem: 101 g + 25.01 g + 5.05 g? 131.06 g 131.1 g 131 g 130 g
Answers: 1
You know the right answer?
If the pressure exerted by a gas at 100°C in a volume of 8.04 L is 3.81 atm, how many moles of gas a...
Questions


Mathematics, 04.11.2020 05:30


Biology, 04.11.2020 05:30


Social Studies, 04.11.2020 05:30

Social Studies, 04.11.2020 05:30

Mathematics, 04.11.2020 05:30


Mathematics, 04.11.2020 05:30

Biology, 04.11.2020 05:30

Spanish, 04.11.2020 05:30

Mathematics, 04.11.2020 05:30


Mathematics, 04.11.2020 05:30



History, 04.11.2020 05:30




