
Chemistry, 18.03.2021 01:40 baileyanne9389
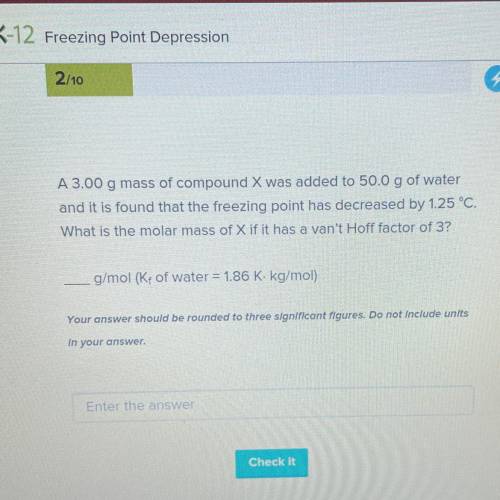
A 3.00 g mass of compound X was added to 50.0 g of water
and it is found that the freezing point has decreased by 1.25 °C.
What is the molar mass of X if it has a van't Hoff factor of 3?
g/mol (K, of water = 1.86 K. kg/mol)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
1.aluminum chloride (alcl3), and sodium hydroxide (naoh) can react to form aluminum hydroxide (al(oh)3) and sodium chloride (nacl). you have 13.4 g of aluminum chloride and 10.0 g of sodium hydroxide. answer the following questions: •what is the balanced equation for this reaction? •if you use all 13.4 g of aluminum chloride, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •if you use all 10.0 g of sodium hydroxide, how many grams of aluminum hydroxide can be formed? work must be shown to earn credit •how many grams of aluminum hydroxide will actually be made? which reagent is limiting? explain your answer.
Answers: 1

Chemistry, 22.06.2019 02:30
Which piece of equipment would me most useful for measuring the volume of some water? a. pan balance b. graduated cylinder c. tweezers d. flask quick
Answers: 2

Chemistry, 22.06.2019 03:30
What is the number of moles of chemical units represented by 9.03x10^24? and how do i show work? (dumb it down )
Answers: 1

Chemistry, 22.06.2019 04:30
Why are people not able to scuba dive in the deep part of the ocean
Answers: 2
You know the right answer?
A 3.00 g mass of compound X was added to 50.0 g of water
and it is found that the freezing point ha...
Questions

Biology, 26.03.2020 19:27

Mathematics, 26.03.2020 19:27



Mathematics, 26.03.2020 19:28





Mathematics, 26.03.2020 19:28






Mathematics, 26.03.2020 19:29

Mathematics, 26.03.2020 19:29

Mathematics, 26.03.2020 19:29

Biology, 26.03.2020 19:29

Mathematics, 26.03.2020 19:29



