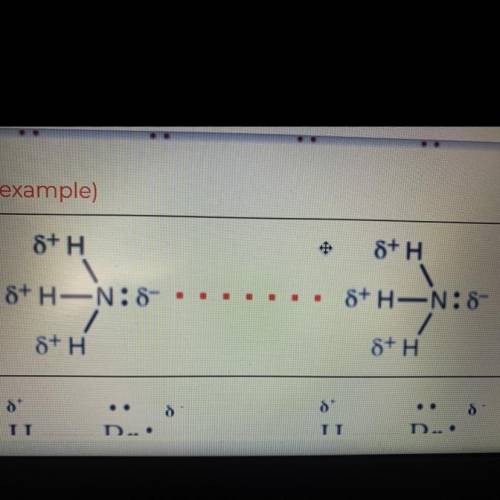
Hydrogen bonding, dipole-dipole, or dispersion forces
And why?
...

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:10
Nitric oxide (no) can be formed from nitrogen, hydrogen and oxygen in two steps. in the first step, nitrogen and hydrogen react to form ammonia: n2(g) + 2 h_2(g) rightarrow 2 nh_3 (g) delta h = -92. kj in the second step, ammonia and oxygen react to form nitric oxide and water: 4 nh_3(g) + 5 o_2(g) rightarrow 4no(g) + 6 h_2o(g) delta h = -905. kj calculate the net change in enthalpy for the formation of one mole of nitric oxide from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 1


Chemistry, 22.06.2019 20:00
Which of the following would not diffuse through the plasma membrane by means of simple diffusion? 1 oxygen 2 glucose 3 a steroid hormone 4 a lipid soluble vitamin
Answers: 3

You know the right answer?
Questions




Mathematics, 18.12.2020 21:10



English, 18.12.2020 21:10



Mathematics, 18.12.2020 21:10

Mathematics, 18.12.2020 21:10


Mathematics, 18.12.2020 21:10

Mathematics, 18.12.2020 21:10





History, 18.12.2020 21:10

Business, 18.12.2020 21:10