Chemistry, 18.03.2021 01:50 taylor3932
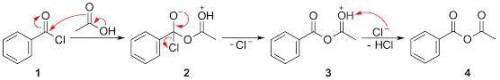
Draw a mechanism for this reaction. In the reaction scheme, an acyl chloride reacts with an acid to give an acid anhydride. The acyl chloride contains a central C atom that has a double bond to O, a single bond to Cl, and a single bond to a benzene ring. The acid is CH3COH with an O atom double-bonded to the second (from left to right) carbon. The produced anhydride is COCCH3 with a benzene ring attached to the first (from left to right) carbon and an O atom double-bonded to the firth and the second carbons.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 13:00
What happens to the average kinetic energy of a gas when the particles of the gas collide against each other at a constant temperature and volume? explain your answer.
Answers: 3

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

Chemistry, 22.06.2019 15:30
How does a large body of water, such as the ocean, influence climate?
Answers: 1

Chemistry, 23.06.2019 00:30
Balance the following reaction. as2s3 + 9o2 → 2as2o3 + so2
Answers: 2
You know the right answer?
Draw a mechanism for this reaction. In the reaction scheme, an acyl chloride reacts with an acid to...
Questions





History, 18.03.2021 02:40

Mathematics, 18.03.2021 02:40

Mathematics, 18.03.2021 02:40

Mathematics, 18.03.2021 02:40




Mathematics, 18.03.2021 02:40

Mathematics, 18.03.2021 02:40





Social Studies, 18.03.2021 02:40