
Chemistry, 18.03.2021 02:00 boogerbuttday
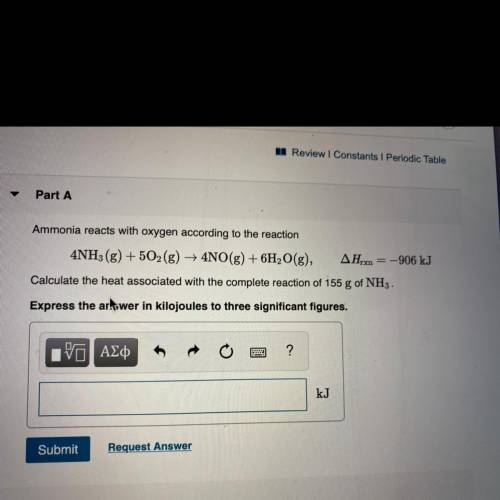
Ammonia reacts with oxygen according to the reaction
4NH3(g) + 5O2(g) + 4NO(g) + 6H2O(g), AHrx = -906 kJ
Calculate the heat associated with the complete reaction of 155 g of NH3 .
Express the artswer in kilojoules to three significant figures.
Se
ΑΣΦ
?
kJ
her
9.pdf
Submit
Request Answer
Part B

Answers: 2
Another question on Chemistry



Chemistry, 23.06.2019 03:00
What happens in the particles of a gas when the gas is compressed
Answers: 1

Chemistry, 23.06.2019 06:30
Which of these natural resources is non-renewable a.corn b.wind c.geothermal d.natural gas
Answers: 2
You know the right answer?
Ammonia reacts with oxygen according to the reaction
4NH3(g) + 5O2(g) + 4NO(g) + 6H2O(g), AHrx = -9...
Questions

Mathematics, 20.09.2020 01:01

Geography, 20.09.2020 01:01

Mathematics, 20.09.2020 01:01

Social Studies, 20.09.2020 01:01

Spanish, 20.09.2020 01:01


Geography, 20.09.2020 01:01


Mathematics, 20.09.2020 01:01

Biology, 20.09.2020 01:01

Mathematics, 20.09.2020 01:01

Mathematics, 20.09.2020 01:01


Mathematics, 20.09.2020 01:01



Computers and Technology, 20.09.2020 01:01

Social Studies, 20.09.2020 01:01

English, 20.09.2020 01:01

English, 20.09.2020 01:01



