
Chemistry, 18.03.2021 02:10 krystlemiller11211
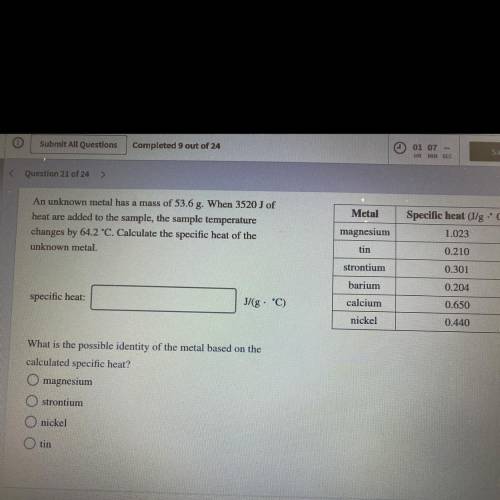
An unknown metal has a mass of 53.6 g. When 3520J of heat are added to the sample, the sample temp. changes by 64.2°C. Calculate specific heat of the unknown metal. what is the metal?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:30
You have 125g of a certain seasoning and are told that it contains 76.0 g of salt what is the percentage of salt by mass in this seasoning
Answers: 1

Chemistry, 22.06.2019 00:30
In numbering carbon atoms in the parent chain of a hydrocarbon, why would you number from right to left, rather than left to right
Answers: 1

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 09:00
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2
You know the right answer?
An unknown metal has a mass of 53.6 g. When 3520J of heat are added to the sample, the sample temp....
Questions






English, 06.11.2019 21:31


Mathematics, 06.11.2019 21:31

History, 06.11.2019 21:31


Biology, 06.11.2019 21:31


Health, 06.11.2019 21:31

Mathematics, 06.11.2019 21:31


Computers and Technology, 06.11.2019 21:31



Medicine, 06.11.2019 22:31



