
Chemistry, 18.03.2021 02:40 HalpMahOnMahH0meW0rk
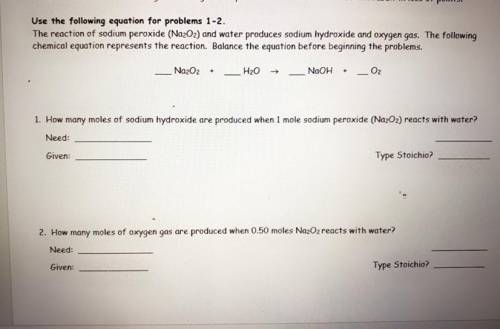
the reaction of sodium peroxide (Na2O2) and water produces sodium hydroxide and oxygen gas. the following chemical equation represents the reaction. balance the equation before beginning the problems. ( use the equation for problems one and two )

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 10:30
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1


Chemistry, 22.06.2019 22:00
Pls ill give u brainliest which of the following is true about science? 1. political conditions are unable to influence it. 2. economic concerns may prevent it from solving problems.
Answers: 2
You know the right answer?
the reaction of sodium peroxide (Na2O2) and water produces sodium hydroxide and oxygen gas. the foll...
Questions

History, 02.07.2019 10:00



History, 02.07.2019 10:00

English, 02.07.2019 10:00



History, 02.07.2019 10:00

History, 02.07.2019 10:00

History, 02.07.2019 10:00


Health, 02.07.2019 10:00

Mathematics, 02.07.2019 10:00

History, 02.07.2019 10:00


Mathematics, 02.07.2019 10:00


History, 02.07.2019 10:00

History, 02.07.2019 10:00

History, 02.07.2019 10:00



