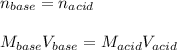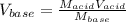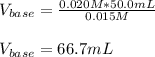Chemistry, 18.03.2021 02:50 ldibut2003
For the titration of 50.0 mL of .020M HI with 0.015 M of NaOH, graph pH versus milliliters of base added from 0-100 mL. How many milliliters of NaOH are added at the equivalence point?

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 3

Chemistry, 22.06.2019 18:30
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1

Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2

Chemistry, 22.06.2019 23:00
What does a numerical subscript following an element in a chemical formula mean?
Answers: 1
You know the right answer?
For the titration of 50.0 mL of .020M HI with 0.015 M of NaOH, graph pH versus milliliters of base a...
Questions

History, 20.02.2021 01:00

Spanish, 20.02.2021 01:00



Mathematics, 20.02.2021 01:00

Biology, 20.02.2021 01:00


Mathematics, 20.02.2021 01:00

Mathematics, 20.02.2021 01:00

English, 20.02.2021 01:00

Mathematics, 20.02.2021 01:00

Mathematics, 20.02.2021 01:00



Mathematics, 20.02.2021 01:00

Mathematics, 20.02.2021 01:00

Mathematics, 20.02.2021 01:00


Mathematics, 20.02.2021 01:00

Mathematics, 20.02.2021 01:00