

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 14:30
Urea, co(nh2)2, is manufactured on a large scale for use in producing urea-formaldehyde plastics and as a fertilizer. what is the maximum mass of urea that can be manufactured from the co2 produced by combustion of 1.00 x 104 grams of co2?
Answers: 1

Chemistry, 22.06.2019 06:30
The minerals found in bones are deposited by living cells called
Answers: 1

Chemistry, 23.06.2019 03:00
Can someone me out on this question for my national 5 chemistry homework
Answers: 1

Chemistry, 23.06.2019 08:40
The activation energy for this reaction is 75 kj·mol–1. the enzyme catalase (found in blood) lowers the activation energy to 8.0 kj·mol–1. at what temperature would the non-catalyzed reaction need to be run to have a rate equal to that of the enzyme-catalyzed reaction at 25°c?
Answers: 2
You know the right answer?
The concentration of Mg2+ in seawater is 0.052 M. At what pH will 76% of the Mg2+ be precipitated as...
Questions





History, 03.02.2020 18:59





Biology, 03.02.2020 18:59


Mathematics, 03.02.2020 18:59

Mathematics, 03.02.2020 18:59


Social Studies, 03.02.2020 18:59

History, 03.02.2020 18:59

Geography, 03.02.2020 18:59

Biology, 03.02.2020 18:59

Mathematics, 03.02.2020 18:59

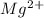 will be precipitated as the hydroxide salt.
will be precipitated as the hydroxide salt.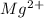 has precipitated out , 24% remains out.
has precipitated out , 24% remains out.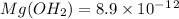
![[Mg^{2}^{+}] =\frac{24}{100}\times 0.052](/tpl/images/1200/9004/3cfbf.png)

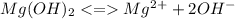
![Ksp = [Mg^2^+][OH^-]](/tpl/images/1200/9004/4a0be.png)
![8.9\times 10^-^1^2= 1.248\times10^-^2\times[OH]^-](/tpl/images/1200/9004/8a6a7.png)
![[OH]^- = 7.131\times 10^-^1^0](/tpl/images/1200/9004/e943a.png)
![[H^+]=\frac{Kw}{[OH]^-}](/tpl/images/1200/9004/234a2.png) ( where Kw is the ionic product of the water)
( where Kw is the ionic product of the water)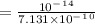

![pH=-Log[H^+]](/tpl/images/1200/9004/3ca39.png)
![-Log[1.402\times10^-^5]](/tpl/images/1200/9004/c76b6.png)



