
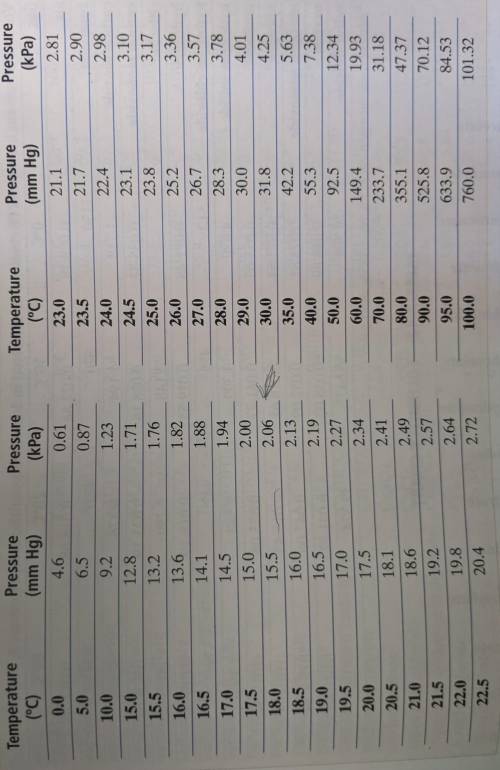
Can someone please help? This was due yesterday. The Appendix Table A-8 is linked in a photo. A certain mass of oxygen was collected over water when potassium chlorate was decomposed by heating. The volume of the oxygen sample collected was 720. mL at 25.0° C and a barometric pressure of 755 torr. What would the volume of the oxygen be at STP? (Hint: First calculate the partial pressure of the oxygen, using Appendix Table A-8. Then use the combined gas law.)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1

Chemistry, 23.06.2019 07:00
Choose the correct statement about licensed veterinarians in the united states. a. they must be certified by the avma. b. they can treat all nonhuman animals. c. they can can treat only animals specified on the license. d. they must choose a specialty.
Answers: 2


Chemistry, 23.06.2019 15:00
An isotope undergoes radioactive decay by emitting radiation that has no mass. what other characteristic does the radiation have?
Answers: 3
You know the right answer?
Can someone please help? This was due yesterday. The Appendix Table A-8 is linked in a photo.
A cer...
Questions

Mathematics, 01.08.2019 08:10


Mathematics, 01.08.2019 08:10

English, 01.08.2019 08:10





Mathematics, 01.08.2019 08:10

Mathematics, 01.08.2019 08:10



Mathematics, 01.08.2019 08:10


Mathematics, 01.08.2019 08:10


English, 01.08.2019 08:10

Mathematics, 01.08.2019 08:10




