
Chemistry, 18.03.2021 03:10 biancabahena04
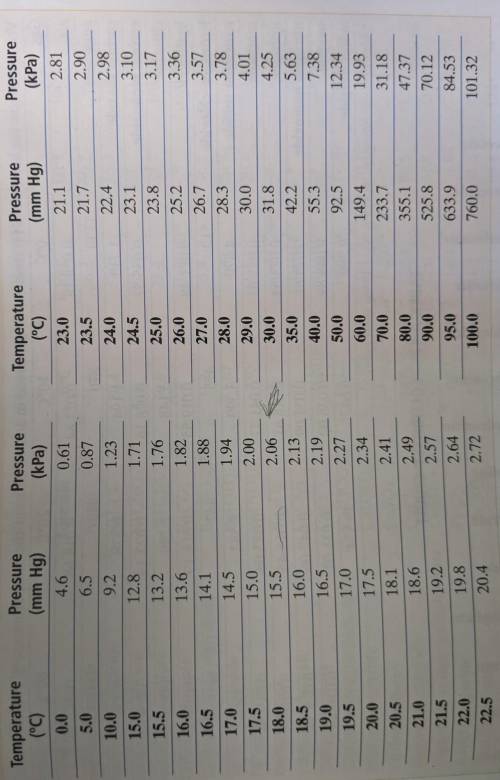
Can someone please help? This was due yesterday. The Appendix Table A-8 is linked in a photo. And do it right please. Don't say "eXpLaNaTiOn iS iN tHe FiLe" like someone else did. No explain it here. A certain mass of oxygen was collected over water when potassium chlorate was decomposed by heating. The volume of the oxygen sample collected was 720. mL at 25.0° C and a barometric pressure of 755 torr. What would the volume of the oxygen be at STP? (Hint: First calculate the partial pressure of the oxygen, using Appendix Table A-8. Then use the combined gas law.)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 20:20
Which symbol can be used to indicate the pressure at which a chemical reaction is carried out? 25°c 2 atm pa
Answers: 2

Chemistry, 22.06.2019 22:00
4.25g sample of solid ammonium nitrate dissolves in 60.0g of water in a coffee-cup calorimeter, the temperature drops from 22.0 c to 16.9 c. assume that the specific heat of the solution is the same as that of pure water. calculate delta(h) (in kj/mol nh4no3) for the solution proces.
Answers: 2

Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3

Chemistry, 23.06.2019 00:00
What is the approximate mass of 25 cm3 of silver, if the density is 10.5 g/cm3? a. 0.42 g b. 2.4 g c. 42 g d. 260 g
Answers: 1
You know the right answer?
Can someone please help? This was due yesterday. The Appendix Table A-8 is linked in a photo. And do...
Questions

Mathematics, 27.06.2019 22:30


History, 27.06.2019 22:30

English, 27.06.2019 22:30

English, 27.06.2019 22:30



Mathematics, 27.06.2019 22:30

Mathematics, 27.06.2019 22:30

Mathematics, 27.06.2019 22:30

Mathematics, 27.06.2019 22:30





Biology, 27.06.2019 22:30


History, 27.06.2019 22:30




