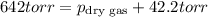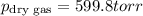Chemistry, 18.03.2021 03:10 superfly903
A sample of gas is collected over water at a temperature of 35.0◦C when the barometric pressure reading is 642 torr. What is the partial pressure of the dry gas, given PH2O = 42.2 torr? Answer in units of torr.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1

Chemistry, 22.06.2019 12:00
What does a complete balanced chemical equation include? a. exothermic coefficients b. endothermic coefficients c. valence electrons d. molar coefficients
Answers: 1


Chemistry, 22.06.2019 16:00
How could a student test the effect of removing heat from a gas that is stored in a sealed container? what must occur in order for matter to change states?
Answers: 2
You know the right answer?
A sample of gas is collected over water at a temperature of 35.0◦C when the barometric pressure read...
Questions


Mathematics, 24.06.2019 00:00










Mathematics, 24.06.2019 00:00

Mathematics, 24.06.2019 00:00

Business, 24.06.2019 00:00




Physics, 24.06.2019 00:00


Mathematics, 24.06.2019 00:00

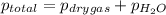
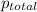 =total pressure of gases = 642 torr
=total pressure of gases = 642 torr
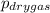 = partial pressure of dry gas = ?
= partial pressure of dry gas = ?
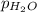 = partial pressure of water = 42.2 torr
= partial pressure of water = 42.2 torr