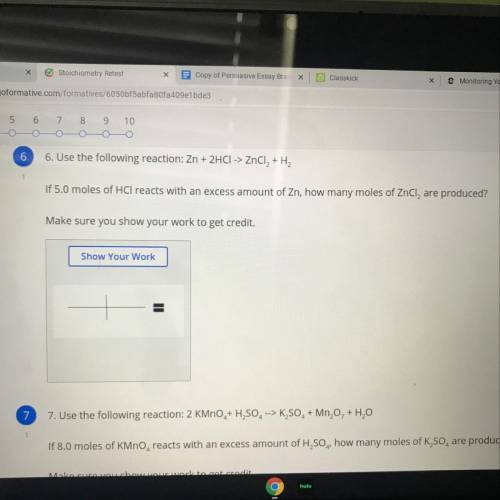
Use the following reaction:
Zn + 2HCI -> ZnCl2 + H2
If 5.0 moles of HCl reacts with an exc...

Chemistry, 18.03.2021 03:20 zairaefh3200
Use the following reaction:
Zn + 2HCI -> ZnCl2 + H2
If 5.0 moles of HCl reacts with an excess amount of Zn, how many moles of ZnCl, are produced?
Make sure you show your work to get credit.
Show Your Work

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 20:00
I’m an electrically neutral atomic any element, there are equal numbers of
Answers: 2

Chemistry, 23.06.2019 04:20
The reaction below shows a system in equilibrium. how would a decrease in temperature affect this reaction? a. the rate of formation of the gases would increase. b. the equilibrium of the reaction would shift to the left. c. the equilibrium would shift to cause the gases to sublime into solids. d. the chemicals on the left would quickly form the chemical on the right.
Answers: 1

Chemistry, 23.06.2019 04:20
The lewis diagrams for magnesium and fluorine are shown below. what is the correct chemical formula for magnesium fluoride? a. mgf b. mg2f c. mgf2 d. mg2f3
Answers: 1

You know the right answer?
Questions




Social Studies, 14.07.2020 20:01


History, 14.07.2020 20:01



History, 14.07.2020 20:01



Mathematics, 14.07.2020 20:01


Mathematics, 14.07.2020 20:01


Mathematics, 14.07.2020 20:01

History, 14.07.2020 20:01


SAT, 14.07.2020 20:01

Mathematics, 14.07.2020 20:01



