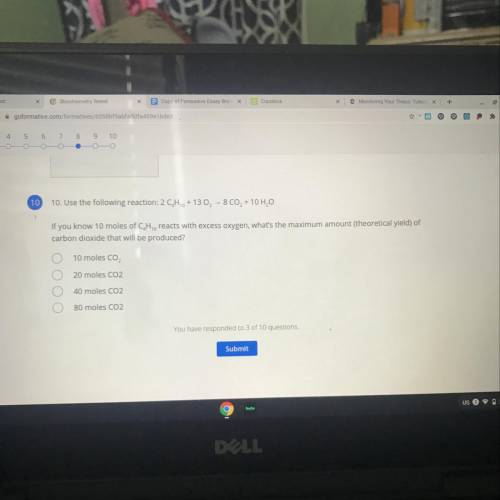
Use the following reaction:
2 CH2 + 13 O2 - 8 CO2 + 10 H2O
If you know 10 moles of C, H, reac...

Chemistry, 18.03.2021 03:30 anonymousanon
Use the following reaction:
2 CH2 + 13 O2 - 8 CO2 + 10 H2O
If you know 10 moles of C, H, reacts with excess oxygen, what's the maximum amount (theoretical yield) of
carbon dioxide that will be produced?
A. 10 moles CO,
B. 20 moles CO2
C. 40 moles CO2
D. 80 moles CO2

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 2

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 06:00
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2

You know the right answer?
Questions




Mathematics, 05.02.2020 03:46


Computers and Technology, 05.02.2020 03:46

Health, 05.02.2020 03:46




History, 05.02.2020 03:46

Mathematics, 05.02.2020 03:46


Advanced Placement (AP), 05.02.2020 03:46


Physics, 05.02.2020 03:46

Mathematics, 05.02.2020 03:46



Mathematics, 05.02.2020 03:46



