
Chemistry, 18.03.2021 07:20 makaylagrandsta
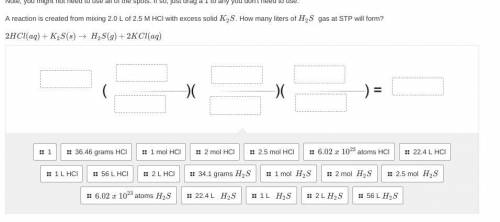
Please only answer w help and i will give you brainliest. <3 A reaction is created from mixing 2.0 L of 2.5 M HCl with excess solid K2S. How many liters of H2S gas at STP will form?
2HCl(aq)+K2S(s)→ H2S(g)+2KCl(aq)
(picture below)
-
C3H8+5O2 → 3 CO2 +4H2O
If 33.6 liters of O2 gas at STP react with 55 grams of C3H8, what is the limiting reactant? How much water can be formed (in grams)?
thank you!!!

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 01:30
Agas is contained in a thick walled balloon when the pressure changes from 1.21 atm to 2.52 the volume changes from 3.75 l to 1.72 l and the temperature change from 293k to blank k
Answers: 3

Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 05:20
Temperature is _related to the average kinetic energy of a gas. inversely directly not disproportionally
Answers: 1

You know the right answer?
Please only answer w help and i will give you brainliest. <3 A reaction is created from mixing 2....
Questions











Mathematics, 19.11.2019 18:31




Mathematics, 19.11.2019 18:31



Computers and Technology, 19.11.2019 18:31





