
Chemistry, 18.03.2021 09:10 xelynncaldera
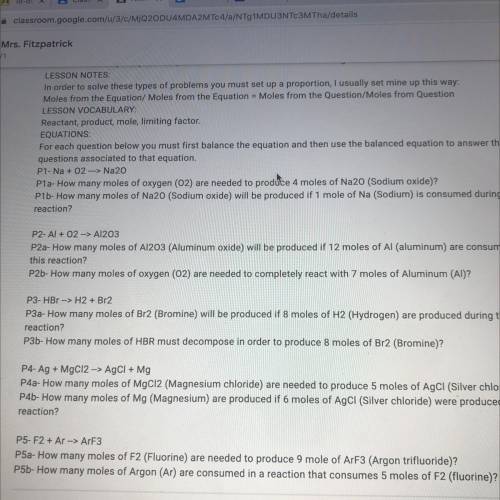
P5-F2 + Ar --> ArF3
P5a- How many moles of F2 (Fluorine) are needed to produce 9 mole of ArF3 (Argon trifluoride)?
P5b- How many moles of Argon (Ar) are consumed in a reaction that consumes 5 moles of F2 (fluorine)?
I need the answers for the p5 part please help

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2



Chemistry, 22.06.2019 22:30
The diagram shows the relationship between scientific disciplines.the names of some scientific disciplines have been removed from the boxes. which scientific discipline belongs in the blue box? a.physics b.biology c.chemistry d.metallurgy
Answers: 2
You know the right answer?
P5-F2 + Ar --> ArF3
P5a- How many moles of F2 (Fluorine) are needed to produce 9 mole of ArF3 (A...
Questions





English, 26.06.2019 11:00












Mathematics, 26.06.2019 11:00


English, 26.06.2019 11:00



