
Chemistry, 19.09.2019 12:10 aaminohasan142
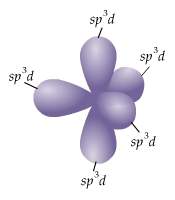
Which of the following clusters of orbitals could form a shape similar to that shown here (figure 3) in the valence shell of an isolated atom or one about to enter into bonding with other atoms?
a) five sp³d
b) three sp² and one p orbital

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 08:00
Identify a strong intermolecular force of attraction between an alcohol
Answers: 1

Chemistry, 22.06.2019 09:00
The diagram below shows a cell placed in a solution.a cell is shown placed inside a beaker. it is labeled cell. the solution inside the beaker is labeled 40% salt solution and the solution inside the cell is labeled 20% salt solution.only water is allowed to move in and out of the cell. what will most likely happen to the cell? it will expand as water moves out of it. it will shrink as water moves out of it.it will expand as water moves into it. it will shrink as water moves into it.
Answers: 2

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2

Chemistry, 23.06.2019 01:30
Which statement accurately represents the arrangement of electrons in bohr’s atomic model?
Answers: 2
You know the right answer?
Which of the following clusters of orbitals could form a shape similar to that shown here (figure 3)...
Questions

Mathematics, 04.06.2020 00:01

Mathematics, 04.06.2020 00:01


Mathematics, 04.06.2020 00:01



Mathematics, 04.06.2020 00:01


Mathematics, 04.06.2020 00:01






History, 04.06.2020 00:01

Mathematics, 04.06.2020 00:01

Mathematics, 04.06.2020 00:01

Mathematics, 04.06.2020 00:01

History, 04.06.2020 00:01

Mathematics, 04.06.2020 00:01



