
Chemistry, 18.03.2021 17:40 itz0nlyheav
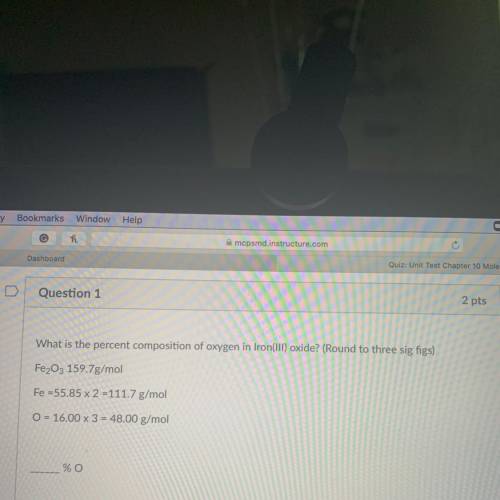
What is the percent composition of oxygen in Iron(III) oxide? (Round to three sig figs)
Fe2O3 159.7g/mol
Fe =55.85 x 2 =111.7 g/mol
O = 16.00 x 3 = 48.00 g/mol
_% O

Answers: 2
Another question on Chemistry

Chemistry, 23.06.2019 04:00
Achemical reaction is done in the setup shown , resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 2


Chemistry, 23.06.2019 06:30
The molar mass of cu is 63.55 g/mol. the number of grams of cu produced in this reaction is
Answers: 3

Chemistry, 23.06.2019 07:30
If you try to move a piano and are unable to move it, did you perform any work in the scientific sense of the word? yes or no?
Answers: 1
You know the right answer?
What is the percent composition of oxygen in Iron(III) oxide? (Round to three sig figs)
Fe2O3 159.7...
Questions


Mathematics, 08.11.2021 09:20

History, 08.11.2021 09:20

History, 08.11.2021 09:20

Physics, 08.11.2021 09:20



Mathematics, 08.11.2021 09:20

Mathematics, 08.11.2021 09:20


English, 08.11.2021 09:20



Mathematics, 08.11.2021 09:20


Biology, 08.11.2021 09:20

English, 08.11.2021 09:20

History, 08.11.2021 09:20


Mathematics, 08.11.2021 09:20



