
Chemistry, 19.03.2021 01:00 genyjoannerubiera
The face-centered cubic unit cell describing the structure of an ionic compound has an edge length of 207 pm. The lattice points of the unit cell are occupied by anions, and the cations are small enough so that the anions are in contact along the diagonal of the face of the unit cell. What is the ionic radius of the anion (in picometers)

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 17:00
What is the empirical formula of vanadium 1 oxide given that 20.38 grams of vandium combines with oxygen to form 23.58 grams of the oxide
Answers: 1

Chemistry, 21.06.2019 19:30
Determine the number o moles of ions/atoms/particle in the following: 2.50 miles of k2s (let me know how to do)
Answers: 1


Chemistry, 22.06.2019 21:30
How can the periodic table be used to predict the behavior of elements?
Answers: 1
You know the right answer?
The face-centered cubic unit cell describing the structure of an ionic compound has an edge length o...
Questions

Mathematics, 29.12.2019 02:31



Mathematics, 29.12.2019 02:31

Social Studies, 29.12.2019 02:31

Mathematics, 29.12.2019 02:31

History, 29.12.2019 02:31

Biology, 29.12.2019 02:31


English, 29.12.2019 02:31


Spanish, 29.12.2019 02:31



Social Studies, 29.12.2019 02:31



Mathematics, 29.12.2019 02:31


Chemistry, 29.12.2019 02:31

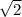 )/4=73.2 pm
)/4=73.2 pm


