
Chemistry, 19.03.2021 06:30 Sanchezj104
2 CH3OH + 3 02 2 CO2 + 4H2O
What is the mass of oxygen (O2) that is required to produce 579 g of carbon dioxide (CO2)?
. Your answer should have three significant figures.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 10:20
In a reaction equation, where are the products located? a.) above the arrow b.) to the right of the arrow c.) to the left of the arrow d.) below the arrow
Answers: 2

Chemistry, 22.06.2019 10:50
How many liters of oxygen gas, at standard temperature and pressure, will react with 35.8 grams of iron metal? 4 fe (s) + 3 o₂ (g) → 2 fe₂o₃ (s)
Answers: 2
You know the right answer?
2 CH3OH + 3 02 2 CO2 + 4H2O
What is the mass of oxygen (O2) that is required to produce 579 g of ca...
Questions

Biology, 27.07.2019 15:40

History, 27.07.2019 15:40

English, 27.07.2019 15:40


Mathematics, 27.07.2019 15:40


Mathematics, 27.07.2019 15:40


Mathematics, 27.07.2019 15:40



Biology, 27.07.2019 15:40





Mathematics, 27.07.2019 15:40

Mathematics, 27.07.2019 15:40


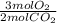 = 19.74 mol O₂
= 19.74 mol O₂

