How many moles of O are in 12.6 mol Fe(NO3)3?
The answer is 113.4, rounded is 113
Explanation...

Chemistry, 19.03.2021 06:40 BLESSEDKIDD1998
How many moles of O are in 12.6 mol Fe(NO3)3?
The answer is 113.4, rounded is 113
Explanation: Just multiply 12.6 mol Fe(NO3)3 by 9 ( 9 is the number of O atoms in the equation)

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 18:00
Balance this equation: n2 + h2 > nh3, write the following molar ratios: n2 / n2 / nh3 h2 /
Answers: 1

Chemistry, 21.06.2019 21:10
Identify one disadvantage to each of the following models of electron configuration: dot structures arrow and line diagrams written electron configurations type in your answer below.
Answers: 1

Chemistry, 22.06.2019 04:00
The continuous release of nuclear energy caused when one fission reaction triggered more nuclear reactions is a
Answers: 3

Chemistry, 22.06.2019 18:00
The human activities in two locations are described below: location a: rampant use of plastic containers location b: excessive use of pesticides and fertilizers which statement is most likely true? location a will have poor air quality because plastic is biodegradable. location a will experience water scarcity because plastic absorbs moisture. the population of honeybees will increase in location b because production of crops will increase. the population of fish in location b will decrease because the water is contaminated.
Answers: 1
You know the right answer?
Questions

Mathematics, 08.06.2021 20:40

Physics, 08.06.2021 20:40





Computers and Technology, 08.06.2021 20:40

English, 08.06.2021 20:40





Mathematics, 08.06.2021 20:40

Arts, 08.06.2021 20:40

Mathematics, 08.06.2021 20:40

Mathematics, 08.06.2021 20:40

English, 08.06.2021 20:40

Social Studies, 08.06.2021 20:40

Mathematics, 08.06.2021 20:40

Mathematics, 08.06.2021 20:40

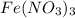
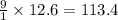 moles of O atoms
moles of O atoms

