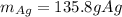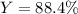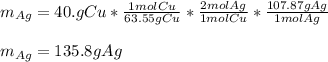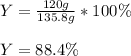Chemistry, 19.03.2021 14:00 squawk1738
Cu + 2AgNO3 → Cu(NO3)2 + 2Ag If a 40. g sample of Copper is used with an excess of silver nitrate, calculate the theoretical yield of silver. What is the percent yield of the silver, if 120 g was collected?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:00
Most materials are not magnetic because their magnetism has worn off. their magnetic domains are arranged randomly. they lack magnetic fields. earth’s heat has destroyed their magnetism.
Answers: 1


Chemistry, 22.06.2019 17:30
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2

Chemistry, 22.06.2019 23:30
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1
You know the right answer?
Cu + 2AgNO3 → Cu(NO3)2 + 2Ag
If a 40. g sample of Copper is used with an excess of silver nitrate,...
Questions

Mathematics, 18.10.2021 21:00

Mathematics, 18.10.2021 21:00


English, 18.10.2021 21:00

Mathematics, 18.10.2021 21:00


History, 18.10.2021 21:00





Chemistry, 18.10.2021 21:00

Computers and Technology, 18.10.2021 21:00

English, 18.10.2021 21:00

SAT, 18.10.2021 21:00

Mathematics, 18.10.2021 21:00