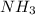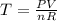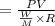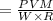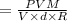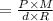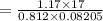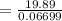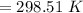Chemistry, 19.03.2021 18:40 Ladarius622
At what temperature (in K) does NH₃ have a density of 0.812 g/L at 1.17 atm?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
The balanced chemical equation for this lab is: 3cucl2(aq) + 2al(s) 3cu(s) + 2alcl3(aq) if 10.5 g copper chloride react with 12.4 g aluminum, what is the limiting reactant?
Answers: 3

Chemistry, 22.06.2019 00:00
How did planetesmals form planets? a. they broke apart into smaller chunks.b. they collided and stuck together.c. they cooled and pulled ice together.d. they began to rotate.
Answers: 1

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

You know the right answer?
At what temperature (in K) does NH₃ have a density of 0.812 g/L at 1.17 atm?...
Questions

Mathematics, 02.12.2020 18:40



Social Studies, 02.12.2020 18:40

Mathematics, 02.12.2020 18:40


Mathematics, 02.12.2020 18:40



Physics, 02.12.2020 18:40

Mathematics, 02.12.2020 18:40

Mathematics, 02.12.2020 18:40


Mathematics, 02.12.2020 18:40




Mathematics, 02.12.2020 18:40

Computers and Technology, 02.12.2020 18:40

English, 02.12.2020 18:40