Chemistry, 19.03.2021 18:20 qveenjordan6456
When a lead acid car battery is recharged by the alternator, it acts essentially as an electrolytic cell in which solid lead(II) sulfate PbSO4 is reduced to lead at the cathode and oxidized to solid lead(II) oxide PbO at the anode. Suppose a current of 96.0A is fed into a car battery for 37.0 seconds. Calculate the mass of lead deposited on the cathode of the battery.

Answers: 3
Another question on Chemistry

Chemistry, 23.06.2019 08:30
Explain how to convert from one unit to another in the metric system.
Answers: 3

Chemistry, 23.06.2019 09:20
Asolution of naoh has a concentration of 25.00% by mass. what mass of naoh is present in 0.250 g of this solution? use the periodic table in the toolbar if needed. 0.0625 g what mass of naoh must be added to the solution to increase the concentration to 30.00% by mass? g
Answers: 2


Chemistry, 23.06.2019 11:00
The lab procedure involves several factors, listed below some were variable and some were constant. label each factor below v for variable ot c for constant
Answers: 1
You know the right answer?
When a lead acid car battery is recharged by the alternator, it acts essentially as an electrolytic...
Questions

Chemistry, 05.03.2021 23:50


Chemistry, 05.03.2021 23:50





Mathematics, 05.03.2021 23:50



Mathematics, 05.03.2021 23:50

Mathematics, 05.03.2021 23:50

Chemistry, 05.03.2021 23:50

Computers and Technology, 05.03.2021 23:50

Social Studies, 05.03.2021 23:50



English, 05.03.2021 23:50

Mathematics, 05.03.2021 23:50

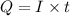
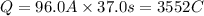
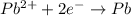
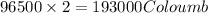 of electricity deposits 1 mole of
of electricity deposits 1 mole of 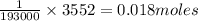 of Pb
of Pb