Writing Equations
1. Potassium reacts with fluorine
2. Potassium nitrate reacts with zinc pho...

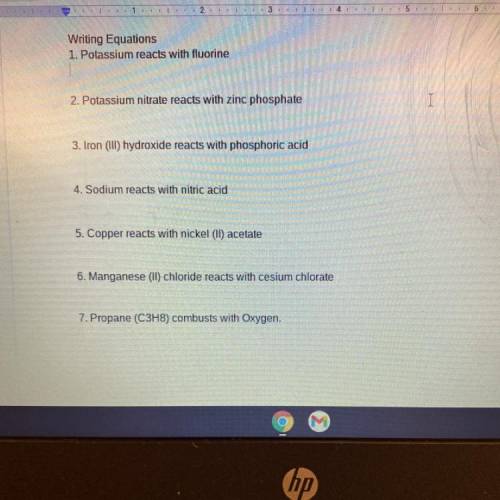
Writing Equations
1. Potassium reacts with fluorine
2. Potassium nitrate reacts with zinc phosphate
3. Iron (III) hydroxide reacts with phosphoric acid
4. Sodium reacts with nitric acid
5. Copper reacts with nickel (II) acetate
6. Manganese (II) chloride reacts with cesium chlorate
7. Propane (C3H8) combusts with Oxygen.
What is the answer to these 7 questions?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Using the periodic table, complete the table to describe each atom. type in your answers.a ? b? c? d? e? f?
Answers: 1

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 11:30
Which statement best describes the flow of energy in this scenario
Answers: 1

Chemistry, 22.06.2019 15:30
The reactions of photosynthesis occur in the of plant cell? a.mitochondria. b. lysosomes. c. chloroplasts. d. chlorophyll
Answers: 1
You know the right answer?
Questions



Spanish, 25.05.2021 21:50


Mathematics, 25.05.2021 21:50

Social Studies, 25.05.2021 21:50





Mathematics, 25.05.2021 21:50



Mathematics, 25.05.2021 21:50

History, 25.05.2021 21:50

Chemistry, 25.05.2021 21:50


History, 25.05.2021 21:50




